Newsletter June 2025
Gene and Cell Therapy: Updates and Innovations
Dear Colleagues,
Gene and Cell Therapies continue transforming cancer treatment in
clinic by offering new ways to target tumors and boost immune responses.
EPO remains a leader in supporting this field through our dedicated
preclinical models and delivery platforms.
Gene and Cell Therapies: Shaping the Future of Oncology
While surgery, chemotherapy, and radiotherapy remain central to cancer treatment, gene and cell therapies have emerged as clinically relevant alternatives. Today, nearly two-thirds of global gene and cell therapy trials focus on cancer. At EPO, these cutting-edge approaches are supported through robust pre-clinical services for both in vitro and in vivo testing.
These therapies work by enhancing anti-tumor immunity or directly targeting tumor cells to inhibit growth, prevent metastasis, induce cell death, or resensitize tumors to conventional therapies. Strategies include T-cell engineering (TCR or CAR T cells), gene editing, RNA-based gene suppression (shRNA, siRNA, miRNA, AS-ODNs), suicide gene therapy, and oncolytic virotherapy.
Advancing Gene and Cell Therapy at EPO
With decades of experience, EPO has contributed to numerous projects involving cytokine delivery, RNA-based therapies, suicide genes, and virotherapy. Recent studies at EPO demonstrated significant anti-tumor effects of clostridium enterotoxin gene therapy in colon and pancreatic cancer models. These efforts utilize advanced in vivo delivery methods, including LNPs, electroporation, jet-injection, and viral vectors (retroviral, lentiviral).
We also conduct TCR and CAR T cell studies to assess anti-tumor activity in CDX and PDX models. We have generated real-time efficacy data in vivo using luciferase-tagged leukemic and lymphoma models. And we are exploring CAR NK cell therapies in neuroblastoma models, expanding the spectrum of cellular immunotherapies.
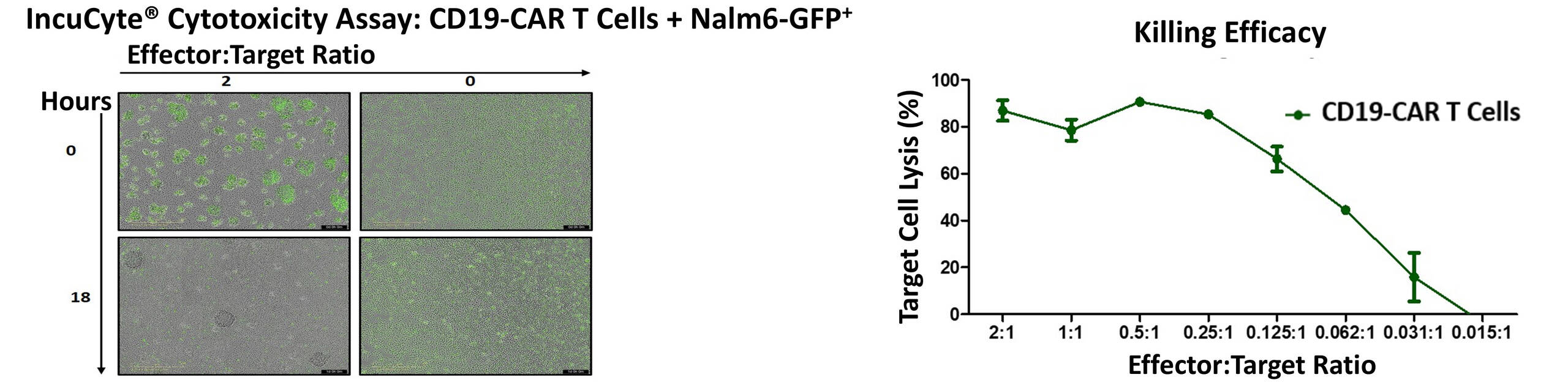
Additionally, EPO supports the production of gene-modified T and NK cells using lentiviral or gammaretroviral transduction. And we complement our Gene and Cell Therapy studies through in vitro assays include real-time cytotoxicity testing with Incucyte® and cytokine profiling via flow cytometry.
Related Selected Publications, which used EPO models and service
- Blood (2024): CRISPR-mediated integration of ζ-deficient CARs into CD3ζ enabled potent antitumor activity in T and NK cells, supporting off- the-shelf CAR-T/NK therapies with reduced GvHD risk and physiological CAR regulation. https://pubmed.ncbi.nlm.nih.gov/38493479/
- Cancers (2021): Claudin-targeted suicide gene therapy using Clostridium perfringens enterotoxin was evaluated in pancreatic cancer PDX models at EPO-Berlin, demonstrating strong oncoleaking-mediated tumor eradication and synergy with chemotherapy. https://pubmed.ncbi.nlm.nih.gov/34503203/
- Eur J Pharm Sci (2021): RIP-encoding suicide nanoplasmids were evaluated in vitro and in EPO-supported neuroblastoma models, showing antitumor efficacy and tolerability—marking the first in vivo application of nanoplasmids for RIP-based gene therapy. https://pubmed.ncbi.nlm.nih.gov/34958884/
- J Immunother Cancer (2021): A mutation-specific TCR targeting MyD88 L265P enabled selective killing of lymphoma cells in vitro and in vivo, supporting precision-engineered TCR-T therapy for B-cell malignancies. https://pubmed.ncbi.nlm.nih.gov/34330762/
- Cancers (2021): CD28-based CAR T cells showed superior trafficking, expansion, and antitumor efficacy over 4-1BB CARs in neuroblastoma and ovarian carcinoma models using a mouse-in-mouse system at EPO-Berlin. https://pubmed.ncbi.nlm.nih.gov/33801448/
- J Control Release (2018): A saporin suicide gene delivered by sapofectosid-enhanced nanoplexes showed antitumor activity and good tolerability in EPO-supported neuroblastoma PDX models. https://pubmed.ncbi.nlm.nih.gov/29481823/
- BMC Cancer (2017): A translation-optimized CPE vector achieved rapid claudin-3/-4-dependent cytotoxicity and selective tumor necrosis in colon cancer PDX models at EPO-Berlin. https://pubmed.ncbi.nlm.nih.gov/28193196/
- Mol Oncol (2014): MIDGE® vectors encoding TNF-α enhanced chemosensitivity and significantly inhibited tumor growth in A375 and melanoma PDX models at EPO-Berlin, supporting clinical development. https://pubmed.ncbi.nlm.nih.gov/24503218/
- Hum Gene Ther Methods (2012): Intratumoral MIDGE®-TNF-α jet- injection yielded high tumor-specific uptake and rapid systemic clearance, supporting safety and tumor-localized gene expression in nonviral gene therapy. https://pubmed.ncbi.nlm.nih.gov/22924532/
- Mol Ther (2008): Jet-injection of shRNA plasmids reversed MDR1- mediated resistance in drug-resistant tumors, restoring doxorubicin sensitivity in vivo in a clinically relevant nonviral delivery approach. https://pubmed.ncbi.nlm.nih.gov/17878902/
For more information, contact Turn on Javascript! to learn how we can support your Gene and Cell Therapy program.
Best regards,
Jens Hoffmann (CEO), Wolfgang Walther (CSO), and Antje Wengner (CSO) EPO Berlin-Buch