Newsletter September 2025
Targeted Radionuclide Therapy (TRT) at EPO
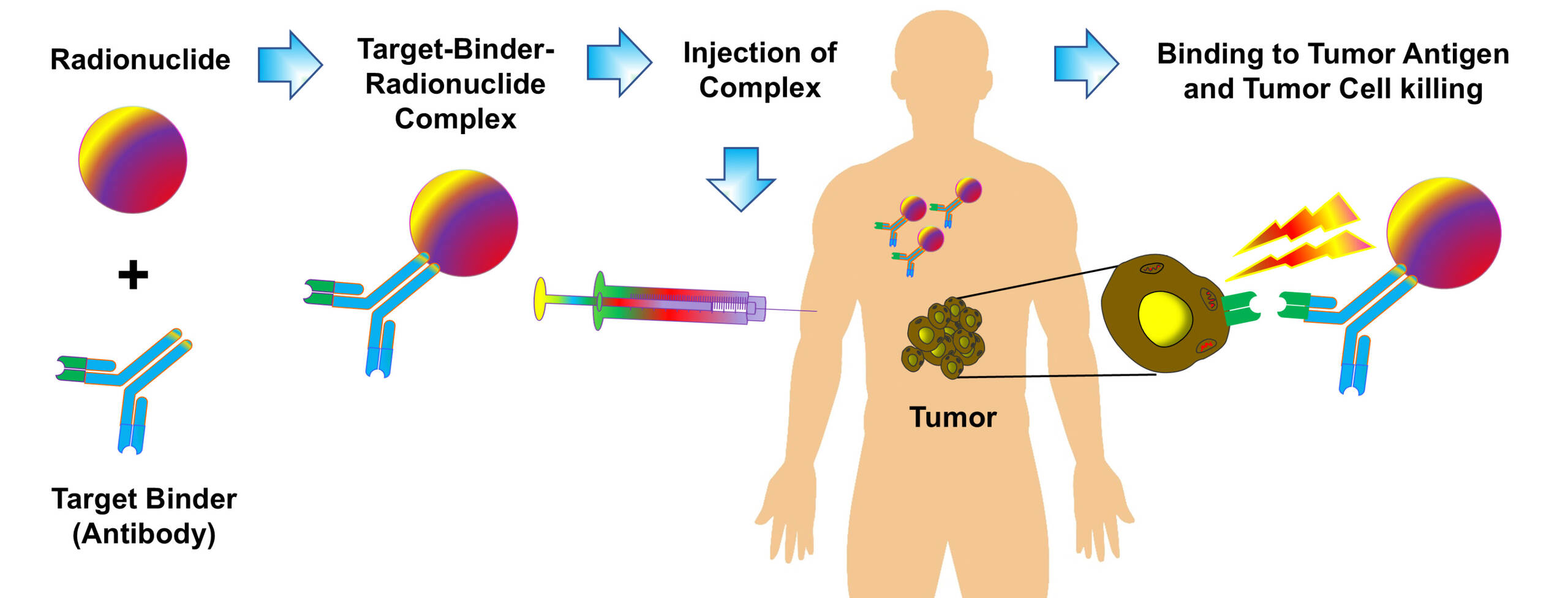
EPO offers advanced capabilities for the assessment of novel radiotherapeutics in oncology research. Our platform supports the development of targeted radionuclide therapies using a range of alpha, beta, and gamma-emitting radioisotopes for precise tumor targeting and effective cancer cell eradication in vitro and in vivo.
Mechanisms of Action
Radiopharmaceuticals are tumor-targeting agents that deliver therapeutic radioisotopes directly to cancer cells, leading to:
- Lethal DNA damage via localized radiation from radionuclide decay
- Direct target blockade of tumor-specific receptors or pathways
- Immune system-mediated cytotoxicity induced by radiolabelled immunomodulators
EPO's Capabilities in TRT Research
EPO offers a robust platform to evaluate efficacy and pharmacokinetics of radiopharmaceuticals:
1. Efficacy Studies
- Conducted in a wide range of patient-derived xenograft (PDX) tumor models
- Available for multiple cancer indications in immunocompromised mice
- Models can be engineered with human immune or stem cell engraftment
2. Biodistribution Studies
- Quantitative analysis of radionuclide distribution across tumor, blood, and organ tissues
- Gamma photon detection using the Wizard 1480 Gamma-Counter (PerkinElmer)
3. PET/CT Imaging
- Qualitative/quantitative analysis and visualization of the of
- radionuclide dissemination in whole body in collaboration with our partner The Charité University of Medicine Berlin, Germany
4. Ex Vivo Tissue Analysis
- Collection and processing of tissues for further molecular and histological analyses
Approved Radionuclides at EPO
EPO is licensed to work with a broad spectrum of radionuclides for preclinical research: 227Th, 223Ra, 225Ac, 213Bi, 3H, 14C, 90Y, 177Lu, 68Ga, 111In, 125I
See also Zboralski et al. Eur J Nucl Med Mol Imag 2022
Partner with EPO
With its established expertise in preclinical oncology and the latest infrastructure for radionuclide research, EPO is your ideal partner for the development and assessment of next-generation targeted radiotherapeutics.
Join us at the EANM 2025 in Barcelona. Let us know if we can schedule a meeting to discuss potential ways how EPO can accelerate the preclinical development of your novel Radiopharmaceuticals. Contact Dr. Sylvia Niebrügge and Dr. Antje Wengner for details.

Best Regards,
Jens Hoffmann (CEO), Wolfgang Walther (CSO), Antje Wengner (CSO)
EPO Berlin-Buch